Psoriasis in a 3-month-old infant with Kawasaki disease
Published Web Location
https://doi.org/10.5070/D36221q3w7Main Content
Psoriasis in a 3-month-old infant with Kawasaki disease
Yi-Chen Liao1, Julia Yu-Yun Lee2
Dermatology Online Journal 15 (11): 10
1. Department of Dermatology, Chi-Mei Hospital, Tainan, Taiwan2. Department of Dermatology, National Cheng Kung University, College of Medicine and Hospital, Tainan, Taiwan. yylee@mail.ncku.edu.tw
Abstract
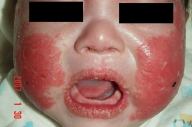
Kawasaki disease (KD) or mucocutaneous lymph node syndrome is a systemic vasculitis of unknown etiology affecting young children. Typical cutaneous manifestations of KD are polymorphous, including maculopapular or morbilliform rash and erythroderma. Occurrence of psoriasis following KD is rare. Herein we report a case of new onset of psoriasis in a 3-month-old that flared after a typical clinical case of KD, manifesting spiking fever, diffuse redness and fissuring of the lips, bilateral conjunctiva injection, injected throat, left cervical lymphadenopathy, erythema and desquamation of the lips, cheeks, hands, feet and perianal area, and a generalized maculopapular eruption. In addition, erythema and induration of the BCG vaccination site and coronary artery dilatation were noted. After fading of the initial rash, the patient developed widespread psoriasiform papules and plaques involving the face and extremities. The cheeks, lips and nail involvement with subunqual hyperkeratosis and pincer nail deformity were particularly striking. The diagnosis of psoriasis was confirmed by skin biopsy. The eruption resolved after one month of topical momentasone furoate treatment. The role of superantigens in KD-associated psoriasis is discussed.
Introduction
Kawasaki disease (KD) is a systemic vasculitis of unknown etiology that usually affects children younger than 5 years. Typical cutaneous manifestations of KD are polymorphous, including erythematous, morbilliform rash and erythroderma. Occurrence of psoriasis during the acute or convalescence phases of KD is rare [1-5]. Herein we report a 3-month-old infant with psoriasis vulgaris-like eruption with onset during the acute phase of KD.
Case report
A previously healthy 3-month-old infant boy was first presented (1/2/2006) with a 2-day history of intermittent high fever associated with cough, rhinorrhea, poor feeding, and irritable crying. Physical examination on admission revealed body temperature 39°C, pulse rate 160/min, respiratory rate 52/min. There was diffuse redness and fissuring of the lips, bilateral conjunctival injection, injected throat, left cervical lymphadenopathy, erythema, and desquamation on the lips, cheeks, hands, feet and perianal area. In addition, erythema and induration of the BCG vaccination site was noted. A generalized maculopapular eruption appeared the next day. The initial tentative diagnosis was fever with acute pharyngitis. Laboratory data were significant for anemia (9.8 g/dL), leukocytosis (26.7 x 103/μL), thrombocytosis (898 x 103/μL), elevated C-reactive protein (139.5 mg/L), and elevated erythrocyte sedimentation rate (34 mm/hr). Urinalysis revealed 40-60 WBC/HPF. Antibiotic treatment with cefazolin and gentamycin were initiated the next day. An echocardiography on 1/4/2006 revealed a trivial mitral valve regurgitation. Spiking fever persisted. Blood and urine cultures yielded negative results. Neither Salmonella or Shigella was isolated from the stool. The clinical and laboratory findings were consistent with KD. Antibiotics were discontinued and he was treated with aspirin and intravenous immunoglobulins (IVIG), 2 g/kg each on 1/5/2006 and 1/7/2006. A follow-up cardiac echo on 1/8 revealed coronary artery dilatation (right coronary artery diameter 0.2 cm, left coronary artery diameter 0.23 cm). Fever subsided after the second dose of IVIG and the maculopapular rash also improved gradually over the next few days. Blood and urine cultures yielded negative results. Sputum cultures isolated a few colonies of S. aureus. No throat culture was obtained. A few colonies of Enterococcus faecalis were isolated from the cerebral spinal fluid. The clinical course was complicated by pneumonia and respiratory distress caused by Chlamydia trachomatis. Ampicillin, Vancomycin, erythromycin, and gentamycin were administered at various time points between 1/6 and 1/16. Pneumonia improved significantly after initiation of erythromycin therapy.
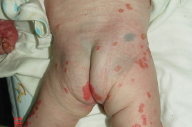
After fading of the initial maculopapular rash, the pediatrician noticed progression of the rash on the limbs and cheeks and requested a dermatology consultation on 1/9/2006, at which time, multiple juicy erythematous papules were found on the lower extremities and face. The eruption worsened progressively, especially on the cheeks, lips, hands, and feet. Dermatology consultation again on 1/17/2006 revealed multiple erythematous papules and small plaques with scales or crust on the extremities and crusted erosions on the cheeks. All nails were involved with subungual hyperkeratosis and pincer nail deformity. Psoriasis or pityriasis lichenoides was suspected. A skin biopsy was performed on 1/24/2006. The patient was discharged on 1/27/2006.
 |  |
| Figure 3 | Figure 4 |
|---|---|
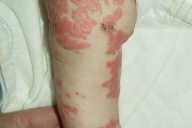
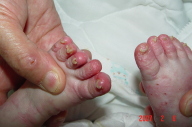
| Figure 3. Extensive psoriatic lesions on the lower extremities. Figure 4. Involvement of digits with subungual hyperkeratosis and pincer nail deformity. | |
 |
| Figure 7 |
|---|
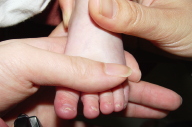
| Resolution of subungual hyperkeratosis pincer nail deformity of the nails. |
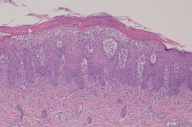
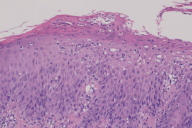
Histopathology of the biopsy specimen revealed changes typical of psoriasis characterized by mounds of parakeratosis containing neutrophils, psoriasiform epidermal hyperplasia with Munro microabscess, diminished granular layer and thinning of supra-papillary plates, and dilated tortuous capillaries.
The skin lesions resolved after a one-month treatment of topical mometasone furoate 0.1 percent cream. The nails also returned to normal gradually.
Discussion
We reported a case of psoriasis occurring in a 3-month-old infant with KD. The diagnosis of KD is based on the criteria proposed by American Heart Association [6] that includes fever of 5 or more days without other explanation and at least 4 of the following 5 criteria: (1) Bilateral, painless, bulbar, non-exudative, conjunctival injection; (2) One of the following mucosal changes: injected or fissured lips, injected pharynx, or strawberry tongue; (3) One of the following changes on the extremities: erythema of the palms or soles, edema of the hands or feet, periungual desquamation; (4) Polymorphous exanthem that can be macular, maculopapular, urticaria, scarletiniform or morbilliform lasting a week; (5) Acute non-suppurative cervical lymphadenopathy (often a painful solitary node>1.5 cm in diameter). Another clinical sign is reactivation of a previous BCG inoculation site manifesting erythema and induration. This reaction has been observed as an early, specific manifestation of KD [7]. The diagnosis of KD in an infant is more difficult than in young children because the manifestations may be subtle.
The differential diagnosis of KD includes infections caused by Streptococcus (scarlet fever, toxic shock syndrome), Staphylococcus (toxic shock syndrome, scalded skin syndrome), Mycoplasma pneumoniae, virus (measles, rubella, roseola infantum, Epstein Barr virus, influenza A and B, adenovirus), and Stevens Johnson syndrome. In the present case, titers for Mycoplasma pneumoniae antibody was 1:80 (normal<40), Chlamydia trachomatis IgM 3.94 (normal ratio<1.0), CMV IgG>250 (normal, 15AU/ml), IgM 0.3 (normal index<0.5). No Streptococcus was isolated from the sputum. The present case met the criteria for the diagnosis of KD.
Spontaneous resolution occurs in most patients, but about 25 percent of the non-treated patients develop a coronary artery aneurysm with an increasing frequency in patients younger than 6 months of age [8]. IVIG and aspirin are required to reduce the possibility of complications and morbidity [8].
Occurrence of psoriasis during the course of KD is rare, but there are at least 16 other reported cases [1-5]. Clinically, KD-associated psoriasis manifests as guttate, plaque, and pustular types [1, 4]. Eberhard et al. [1] documented the first 10 cases (aged 7 to 72 months, mean 12 months). Clinically, the psoriatic eruptions occurred during the acute or convalescent stages of KD. The rash was psoriasiform in 7 patients but pustular in 3. The onset was during the acute stage in 5 patients and during the convalescent stage (4-12 weeks) in the remaining 5. The diagnosis of psoriasis was confirmed by skin biopsy in two patients. Nine of the 10 cases were among a series of 476 cases of KD, representing an incidence of 1.9 percent. After the report by Eberhard et al. [1], 6 more cases were documented [2, 3, 4, 5]. The youngest patient was a 3-month-old [4]. The rash usually resolves in a few months. Only one patient had the eruption persist beyond one year [2]. Our patient was also 3 months of age and the skin lesions, including the severe nail changes, resolved rapidly after low potency topical corticosteroid treatment.
Kobner phenomenon is common in psoriasis; new lesions develop at the site of previous injury. In the present case, after resolution of the initial skin lesions, a new psoriasiform eruption appeared, which was confirmed to be psoriasis. Interestingly, the psoriasis first manifested as recurrence of diffuse erythema with scaling of the cheeks and lips. These findings are consistent with a manifestation of Kobner phenomenon.
The concurrence of KD and psoriasis maybe explained by the fact that both can be triggered by bacterial toxins of streptococcus and staphylococcus [3]. Alternatively, Eberhard et al. [1] postulate that KD and psoriasis are associated through "uncovering" of psoriasis by cytokines released during the acute phase of KD in predisposed individuals [1]. Both KD and guttate psoriasis may be triggered by streptococcus or staphylococcus toxins [9, 10]. These toxins function as superantigens, which are potent activators of CD4+ T cells and can induce a large expansion of unique subsets of Vβ T cells (up to 20% of cells in a given T cell population) and massive production of proinflammatory cytokines independent of antigen specificity [9, 10, 11]. Studies using an animal model for multiple sclerosis show that superantigens can induce severe relapse of diseases and activate autoreactive T cells that are not involved in the initial bout of the disease. Superantigens may also stimulate epitope spreading of disease. The rapid evolution and resolution of the KD-associated psoriatic eruption may reflect the changing levels of cytokines during the course of KD. Because reactivation of a previous BCG inoculation site is an early, specific manifestation of KD, Yokota et al. (ref) examined the potential role of mycobacterial heat shock protein (HSP), and found that HSP65 may be the most potent factor predisposing to Kawasaki disease and that an autoreactivity to the epitope of the human HSP65 homolog may be related to susceptibility to the disease.
In summary, we report a pathology-confirmed case of KD-associated psoriasis during the acute phase of KD. The psoriasiform eruption first appeared on the cheeks, lips and the BCG vaccination site after resolution of the original KD eruption, a finding consistent with a manifestation of Kobner's phenomenon.
References
1. Eberhard BA, Sundel RP, Newburger JW, et al. Psoriatic eruption in Kawasaki disease. J Pediatr. 2000; 137(4):578-580. [PubMed]2. Mizuno Y. Suga Y, Muramatsu S, et al. Psoriasiform and palmoplantar pustular lesions induced after Kawasaki disease. Int J Dermatol. 2006; 45:1080-1082. [PubMed]
3. Yoon SY, Oh ST, Lee JY, Cho BK. Plaque type psoriasiform eruption following Kawasaki disease. Pediatr Dermatol. 2007;24(1):336-337. [PubMed]
4. Menni S, Gualandri L, Boccardi D, et al. Association of pasoriasis-like eruption and Kawasaki disease. J Dermatol. 2006;33(8):571-573. [PubMed]
5. Garty B, Mosseri R, Finkelstein Y. Guttate psoriasis following Kawasaki disease. Pediatr Dermatol 2001; 18:507-508. [PubMed]
6. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease: Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:355 [PubMed]
7. Yokota S, Tsubaki K, Kuriyama T, et al. Presence in Kawasaki disease of antibodies to mycobacterial heat-shock protein HSP65 and autoantibodies to epitopes of human HSP65 cognate antigen.Clin Immunol Immunopathol. 1993 May;67(2):163-70. [PubMed]
8. Rosenfeld EA, Corydon KE, Shulman S. Kawasaki disease in infants less than one year of age. J Pediatr 1995;126:524-9 [PubMed]
9. Abe J, Kotzin BL, Jujo K et al. Selective expansion of T cells expressing T cell receptor variable regions Vbeta2 and Vbeta8 in Kawasaki disease. Proc Natl Acad Sci USA 1992; 89:4066-70. [PubMed]
10. Leung DYM, Travers JB, Norris DA. The role of superanatigens in skin disease. J Invest Dermatol.1995; 105:37-42. [PubMed]
11. Torres BA, Kominsky S, Perrin GQ, et al. Superantigens: The good, the bad, and the ugly. Exp Biol Med. 2001; 226(3):164-176. [PubMed]
© 2009 Dermatology Online Journal